Using Photocatalysis to Easily Break Down 'Forever Chemicals' PFAS
Today, we would like to share a new and notable research breakthrough in the field of environmental science. The news highlights a technology developed by researchers at Rikkyo University and others that uses photocatalysis to easily break down PFAS (per- and polyfluoroalkyl substances) *1, commonly known as 'forever chemicals.'
What is PFAS? *1
PFAS, known for their exceptional durability, are widely used in everyday items*2 such as cookware, food packaging, and clothing due to their resistance to water, oil, and heat.
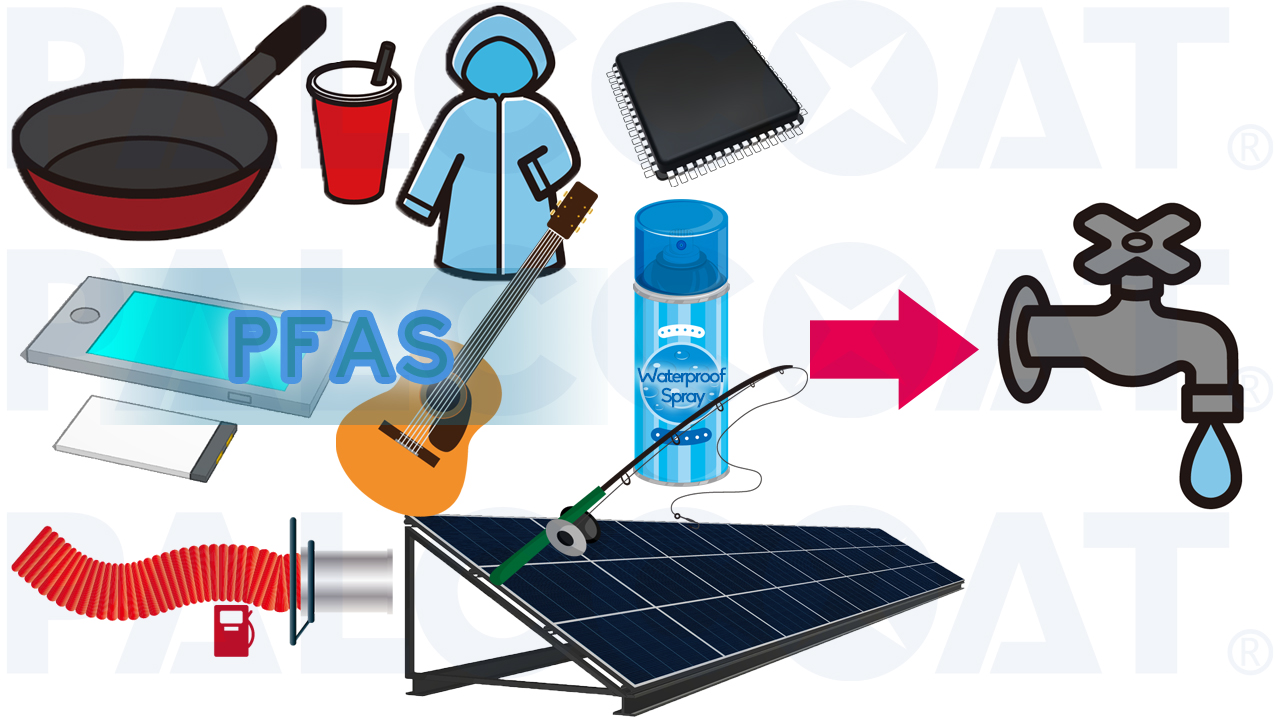
(*2 Coatings for frying pans and pots, lithium-ion batteries, guitar strings, fishing lines, fuel hoses, semiconductor manufacturing, solar panels, tires for snow vehicles, waterproof sprays, and more.)
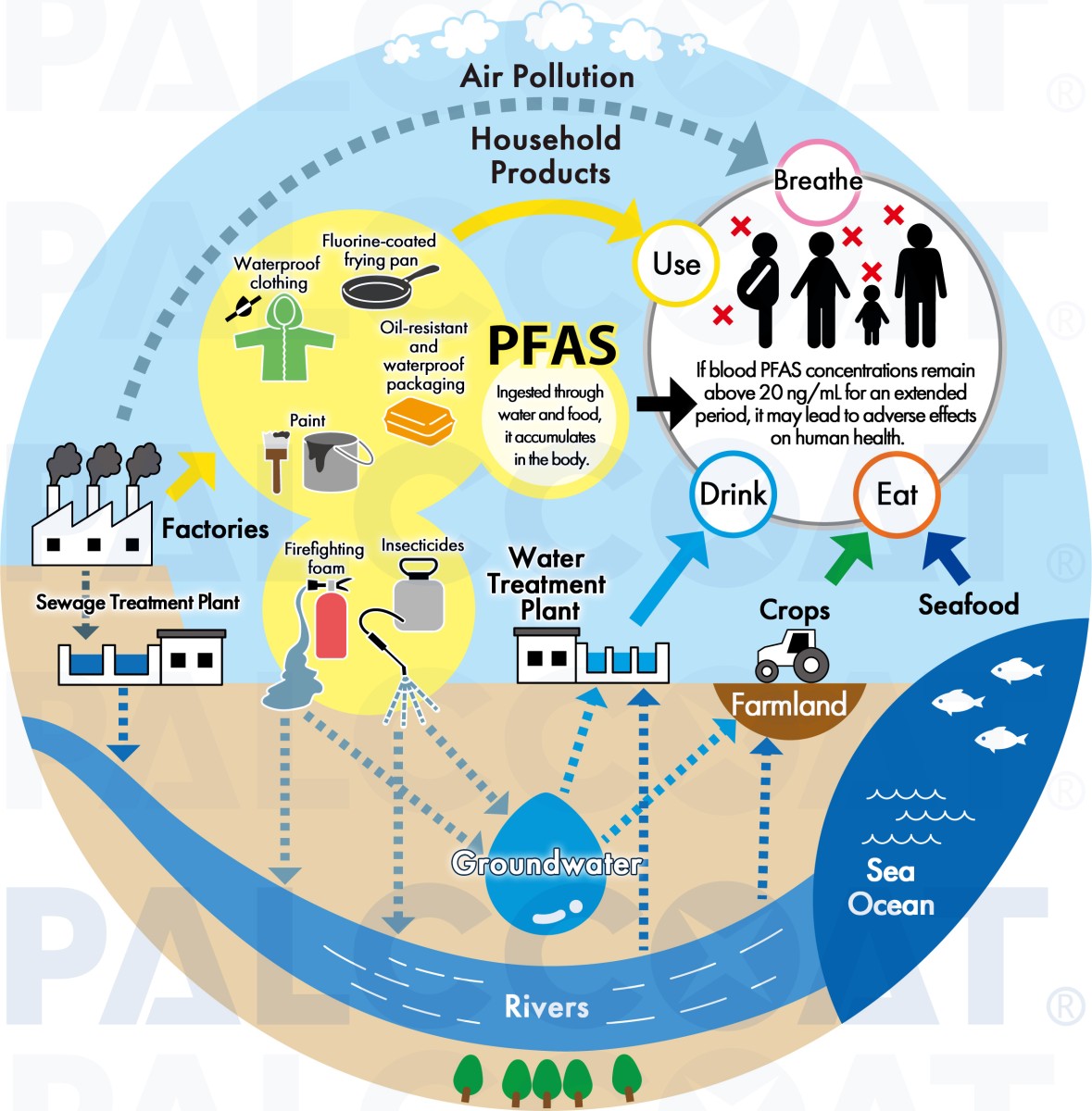
However, these compounds are extremely difficult to break down and persist in the environment, earning the title of "forever chemicals." As a result, PFAS treatment and recycling have become significant environmental challenges.
(Fluoropolymers and more than 10,000 variations, including PFOS, PFOA, and PFHxS, are regulated under the Stockholm Convention.)
Cleavage of C-F Bonds and the Role of Photocatalysts
The Toughness of C-F Bonds
The carbon-fluorine (C-F) bonds in PFAS are among the strongest chemical bonds (about 485 kJ/mol), making it extremely difficult to decompose using traditional oxidation processes. To overcome this, photocatalysts are used to generate high-energy reactive species (e.g., radicals or electrons), which can break the C-F bonds.
Titanium Dioxide (TiO₂)
Titanium dioxide (TiO₂) is widely used to decompose pollutants due to its ability to absorb UV light and trigger strong oxidation reactions. However, breaking C-F bonds requires significant energy, and TiO₂ alone may have limited efficiency for this task.
Cadmium Sulfide (CdS)
Cadmium sulfide (CdS) can utilize visible light, and the reactive species it generates may be more effective at targeting C-F bonds. Specifically, certain radicals and electron states produced by CdS are believed to accelerate the decomposition of C-F bonds.
Comparison of Properties between TiO₂ and CdS
| Property | Titanium Dioxide (TiO₂) | Cadmium Sulfide (CdS) |
|---|---|---|
| Light Absorption | UV (wavelength < 400 nm) | Visible light (wavelength 400-700 nm) |
| Primary Reaction Mechanism | Generates hydroxyl radicals (•OH) with strong oxidizing power | Generates radicals, electrons, and holes (effective under specific conditions) |
| Photocatalytic Reaction Rate | High and stable under many environmental conditions | Efficient under specific reaction conditions |
| Challenges | Requires UV light, limiting visible light use | Needs careful handling due to self-decomposition and toxicity |
The Recent Research Achievement
A research team from Ritsumeikan University has developed a technique for decomposing PFAS using photocatalysts. This technique has the following key features:
- No Heat Required for Decomposition: Traditionally, high temperatures or strong chemicals were necessary, but this method overcomes that challenge.
- Environmentally Friendly: The use of photocatalysts significantly reduces energy consumption.
- Efficiently Breaks C-F Bonds: This technique can transform waste into a more recyclable form.
PFAS is believed to consist of over 10,000 different compounds, some of which are regulated due to manufacturing and usage restrictions. The ability to decompose these substances can significantly reduce the environmental impact during product disposal.
The decomposition of PFAS is one of the major environmental challenges we face today. With breakthroughs like this research, the advancement of science and technology worldwide will become a key element in safeguarding the future of our planet.
Our company is also committed to developing and promoting environmentally friendly products and technologies.
